S140 Interbody System

The S140 Interbody System, used in conjunction with the S100 Pedicle Screw System, provides a comprehensive solution for stabilization of the lumbar spine. Manufactured by Calvary Spine, LLC from PEEK-OPTIMA®†, these interbody devices were designed for PLIF/TLIF and either unilateral or bilateral placement.

Anatomic design features include a tapered “bullet nose” geometry, curved contour, and 8-degree angle of lordosis. Device sizes options range from 7 – 15 mm (anterior height).

PEEK-OPTIMA is a registered trademark of Victrex PLC.
Features and Benefits
01 PEEK-OPTIMA®†
- Proven long-term biocompatibility
- Modulus of elasticity in the range of cortical and cancellous bone.
02 Tapered ‘bullet-nose’ design
- Eases insertion
- Protects against violation of the endplate
03 Inferior and superior surface ridges
- Resists implant migration
- Provides substantial surface area to prevent subsidence
04 Large graft windows and graft volume
- Facilitates bony integration
05 Central I-beam design
- Resists de-formation during placement
- Allows substantial bone graft compartments
06 Curved Contour
- Approximated the geometry of the disk space disc space periphery, allowing contact with the most dense endplate bone
07 8 Degrees of Lordosis
- Allows restoration of segmental sagittal alignment and coronal obliquity
08 Radiographic Markers
- Embedded tantalum pins show implant position on x-ray, without obscuring the view of the healing fusion
09 10 and 25 degree insertion angles
- Permits flexibility in surgical approach, minimizing neural retraction
10 Efficient instrumentation
- Intuitive, ergonomic insertion tools
- Trial spacers to determine optimal implant size and confirm position radiographically
11 Range of Sizes
- 7mm – 15mm anterior heights
PEEK-OPTIMA is a registered trademark of Victrex PLC.
Sizes and Specs
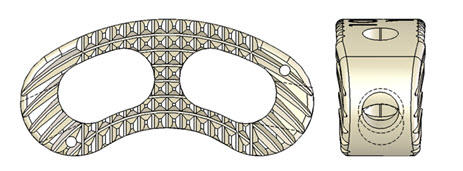
01 Dimensions:
- 11mm by 27mm sizing
- Height options: 7 mm -15 mm (1 mm increments)
02 Substantial area of graft-to-endplate contact and graft compartment
- Graft area: minimum 90mm2
- Graft volume: minimum 630mm3
KYOCERA Medical Technologies, Inc.
