E-MAX Highly Crosslinked Polyethylene

E-MAX is a step forward in the evolution of highly crosslinked polyethylene (XLPE) for orthopedic total joint bearing surfaces.

E-MAX is a step forward in the evolution of highly crosslinked polyethylene (XLPE) for orthopedic total joint bearing surfaces.
- Highly crosslinked
- A proven strategy for reducing in vivo wear [1-10]
- Mechanically annealed
- Eliminates* free radicals without the problems of melting [11,12]
- Vitamin E blended
- Intended to provide oxidative stability over time in vivo [12,13]
Like first-generation XLPE, E-MAX is highly crosslinked to reduce wear. However, E-MAX incorporates two new technologies—mechanical-anneal and Vitamin E—which address known problems with melt-annealed XLPE. Laboratory tests have verified the superior oxidative stability and improved tensile strength and toughness of E-MAX.[14,15]
Learn more about the E-MAX for Hip and E-MAX for Knee.
*Free radicals are eliminated to a level at or near the detection limit of ESR measurement equipment.
- Kurtz S, Medel FJ, MacDonald D, Rimnac CM. In vivo oxidation, oxidation potential, and clinical performance of highly crosslinked UHMWPEs implanted for up to 8 years. 4th International Meeting UHMWPE for arthroplasty: From Powder to Debris 2009; Torino, Italy.
- Digas G, Karrholm J, Thanner J, Herberts P. 5-year experience of highly cross-linked polyethylene in cemented and uncemented sockets: Two randomized studies using radiostereometric analysis. Acta Orthop 2007; 78(6): 746-54.
- D’Antonio JA, Manley MT, Capello WN, et al. Five-year experience with Crossfire highly cross-linked polyethylene. Clin Orthop Relat Res 2005; 441: 143-50.
- Engh CA, Stepniewski AS, Ginn SD, et al. A randomized prospective evaluation of outcomes after total hip Arthroplasty using crosslinked marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty 2006; 21(6): 17-25.
- Olyslaegers C, Defoort K, Simon JP, Vandenberghe L. Wear in conventional and highly cross-linked polyethylene cups: a 5-year follow-up study. J Arthroplasty 2008; 23(4): 489-94.
- Garcia-Rey E, Garcia-Cimbrelo E, Cruz-Pardos A, Ortega-Chamarro J. New polyethylenes in total hip replacement: a prospective, comparative clinical study of two types of liner. J Bone Joint Surg Br 2008; 90(2): 149-53.
- Geerdink CH, Grimm B, Vencken W, Heyligers IC, Tonino AJ. Cross-linked compared with historical polyethylene in THA: An 8-year clinical study. Clin Orthop Relat Res 2009; 467(4): 979-84.
- Glyn-Jones S, Isaac S, Hauptfleisch J, McLardy-Smith P, Murray DW, Gill HS. Does highly cross-linked polyethylene wear less than conventional polyethylene in total hip arthroplasty? A doubleblind, randomized, and controlled trial using roentgen stereophotogrammetric analysis. J Arthroplasty 2008; 23(3): 337-43.
- Kurtz SM, Medel FJ, MacDonald DW, Parvizi J, Kraay MJ, Rimnac CM. Reasons for revision of first-generation highly cross-linked polyethylenes. J Arthroplasty. 2010 Sep;25(6 Suppl):67-74.
- Kurtz SM. Chapter 8 The clinical performance of UHMWPE in knee replacements. In UHMWPE Biomaterials Handbook Second Edition (ed. Kurtz SM). Elsevier: Amsterdam, 2009.
- Bhattacharyya S, Matrisciano L, Spiegelberg S, Harris W, Muratoglu O. Mechanical elimination of residual free radicals in an irradiated UHMWPE rod: advantages over melting. 50th annual meeting of the orthopaedic research society. 2004:1474.
- Wannomae KK, Micheli BR, Lozynsky AJ, Muratoglu OK. A New Method of Stabilizing Irradiated UHMWPE Using Vitamin E and Mechanical Annealing. 11th Congress EFFORT. Madrid, Spain. June 2010.
- Costa L, Bracco P. Chapter 21 Mechanisms of crosslinking, oxidative degradation, and stabilization of UHMWPE. In UHMWPE Biomaterials Handbook Second Edition (ed. Kurtz SM). Elsevier: Amsterdam, 2009.
- Materials Characterization testing. Test report TP0322. On file with KYOCERA Medical Technologies, Inc.
- Cambridge Polymer Group. Analysis of CIMA and E-CIMA Material. Test report dated July 15, 2011. On file with KYOCERA Medical Technologies, Inc.
Background
01 Successes and Setbacks with Highly Crosslinked Polyethylene
Highly crosslinked polyethylene (XLPE) was developed to address the two major factors known to affect polyethylene longevity in vivo: wear and oxidation.[1] While the XLPE process varies among manufacturers, generally it includes: radiation crosslinking for wear resistance and thermal annealing to address oxidation.
02 Proven Wear Reduction—with Drawbacks
Laboratory and clinical studies of first-generation XLPE have verified that radiation crosslinking results in improved wear when compared to conventional* polyethylene.[2-10] However, improved wear and resistance to oxidation come at the expense of mechanical properties.[11-15]
| Drawbacks Associated with First-Generation XLPE | ||
| Process Step | Benefit | Drawback |
|---|---|---|
| Crosslinking ( |
||
| Melt-Anneal ( |
||
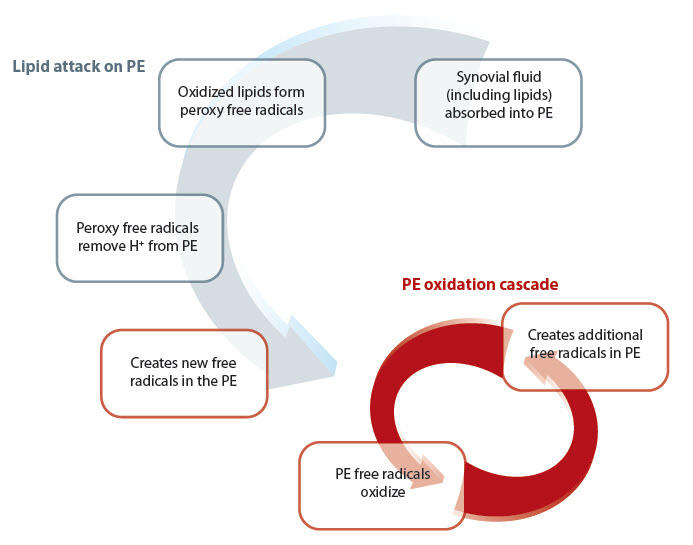
03 Unexpected In Vivo Surface Damage and Oxidation
Several reports of surface damage and catastrophic failure in first-generation XLPE hip liners have been published.[16-19] Researchers have noted pitting, cracking, and surface wear similar to conventional polyethylene.[20-24] More recent retrieval studies of melt-annealed XLPE liners have also revealed unexpected findings regarding oxidation, citing:
- Measurable oxidation potential [23]
- Increased oxidation with longer implantation time [24]
- Evidence that free radicals are re-introduced in vivo [11]
Researchers theorize that the free radicals are introduced when lipids are absorbed into the polyethylene, triggering the oxidation cascade. [11]
Proposed Process for in vivo Oxidation of Melt-Annealed XLPE [11]
*The term “conventional” refers to the control in published studies of XLPE: gamma-sterilized-in-air and, more recently, oxygenless-packaged PE.
- Muratoglu OK. Chapter 13 Highly Crosslinked and Melted UHMWPE. In UHMWPE Biomaterials Handbook Second Edition (ed. Kurtz SM). Elsevier: Amsterdam, 2009.
- Kurtz S, Medel FJ, MacDonald D, Rimnac CM. In vivo oxidation, oxidation potential, and clinical performance of highly crosslinked UHMWPEs implanted for up to 8 years. 4th International Meeting UHMWPE for arthroplasty: From Powder to Debris 2009; Torino, Italy.
- Digas G, Karrholm J, Thanner J, Herberts P. 5-year experience of highly cross-linked polyethylene in cemented and uncemented sockets: Two randomized studies using radiostereometric analysis. Acta Orthop 2007; 78(6): 746-54.
- D’Antonio JA, Manley MT, Capello WN, et al. Five-year experience with Crossfire highly cross-linked polyethylene. Clin Orthop Relat Res 2005; 441: 143-50.
- Engh CA, Stepniewski AS, Ginn SD, et al. A randomized prospective evaluation of outcomes after total hip Arthroplasty using crosslinked marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty 2006; 21(6): 17-25.
- Olyslaegers C, Defoort K, Simon JP, Vandenberghe L. Wear in conventional and highly cross-linked polyethylene cups: a 5-year follow-up study. J Arthroplasty 2008; 23(4): 489-94.
- Garcia-Rey E, Garcia-Cimbrelo E, Cruz-Pardos A, Ortega-Chamarro J. New polyethylenes in total hip replacement: a prospective, comparative clinical study of two types of liner. J Bone Joint Surg Br 2008; 90(2): 149-53.
- Geerdink CH, Grimm B, Vencken W, Heyligers IC, Tonino AJ. Cross-linked compared with historical polyethylene in THA: An 8-year clinical study. Clin Orthop Relat Res 2009; 467(4): 979-84.
- Glyn-Jones S, Isaac S, Hauptfleisch J, McLardy-Smith P, Murray DW, Gill HS. Does highly cross-linked polyethylene wear less than conventional polyethylene in total hip arthroplasty? A doubleblind, randomized, and controlled trial using roentgen stereophotogrammetric analysis. J Arthroplasty 2008; 23(3): 337-43.
- Kurtz SM, Medel FJ, MacDonald DW, Parvizi J, Kraay MJ, Rimnac CM. Reasons for revision of first-generation highly cross-linked polyethylenes. J Arthroplasty. 2010 Sep;25(6 Suppl):67-74.
- Muratoglu OK, Wannomae KK, Rowell SL, et al. Ex Vivo Stability Loss of Irradiated and Melted Ultra-High Molecular Weight Polyethylene. JBJS 2010;92:2809-2816.
- Kurtz SM. Chapter 6 Contemporary total hip arthroplasty: hard-on-hard bearings and highly crosslinked UHMWPE. In UHMWPE Biomaterials Handbook Second Edition (ed. Kurtz SM). Elsevier: Amsterdam, 2009.
- Oral E, Malhi AS, Muratoglu OK. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006; 27(6):917-25.
- Baker DA, Bellare A, Pruitt L. The effects of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. Journal of Biomedical Materials Research 2003; 66A(1): 146-154.
- Rimnac C. On the mechanical properties of UHMWPE. Transactions of the 3rd UHMWPE International Meeting. Polyethylene in Total Joint Replacement Systems: Concerns and Solutions. Madrid, Spain. September 14-15, 2007.
- Tower SS, Currier JH, Currier BH, Lyford KA, Van Citters DW, Mayor MB. Rim cracking of the cross-linked Longevity polyethylene acetabular liner after total hip arthroplasty. JBJS 2007; 89(10): 2212-2217.
- Halley D, Glassman A, Crowninshield RD. Recurrent dislocation after revision total hip replacement with a large prosthetic femoral head. A case report. JBJS 2004; 86(4):827-830.
- Moore KD, Beck PR, Petersen DW, Cuckler JM, Lemons JE, Eberhardt AW. Early failure of a cross-linked polyethylene acetabular liner. A case report. JBJS 2008; 90(11):2499.
- Duffy GP, Wannomae KK, Rowell SL, Muratoglu OK. Fracture of a crosslinked polyethylene liner due to impingement. J Arthroplasty 2009; 24(1): 158.e15-158.e19.
- Bradford L, Baker DA, Graham J, Chawan A, Ries MD, Pruitt LA. Wear and surface cracking in early retrieved highly cross-linked polyethylene. JBJS 2004;86-A(6):1271-82.
- Furmanski J, Kraay MJ, Rimnac CM. Crack Initiation in Retrieved Cross-Linked Highly Cross-Linked Ultrahigh-Molecular-Weight Polyethylene Acetabular Liners: An Investigation of 9 Cases. J Arthroplasty. 2010 Sep 20. [Epub ahead of print]
- Schroder DT, Kelly NH, Wright TM, Parks ML. Retrieved highly crosslinked UHMWPE acetabular liners have similar wear damage as conventional UHMWPE. Clin Orthop Relat Res 2010; 469(2):387-94.
- Kurtz SM, MacDonald D, Brenner E, Medel FJ, Hozack W, Parvizi J, Goldberg V, Kraay M, Stulberg B, Rimnac CM. In vivo oxidation, oxidation potential, and clinical performance of first and second generation highly crosslinked acetabular bearings for THA. Poster No. 1790 54th Annual Meeting of the ORS, 2008.
- Currier BH, Van Citters D., Currier JH, Collier JP. In Vivo Oxidation in Remelted Highly Cross-Linked Retrievals. JBJS 2010; 92(14):2409-18.
Technology Description
E-MAX is vitamin E-blended (E), mechanically-annealed (MA), crosslinked (X) polyethylene.
01 Developed in Conjunction with Renowned Polymer Experts
The E-MAX technology was developed by polymer scientists at Massachusetts General Hospital (MGH) and Cambridge Polymer Group (CPG). Engineers collaborated with MGH and CPG to tailor the material’s mechanical properties to meet hip and knee design requirements.
Each step of the E-MAX manufacturing process was designed to capitalize on lessons learned from the experience with first-generation XLPE.

1 Raw Material
1020 UHMWPE resin is blended with 0.1% (by weight) vitamin E powder.
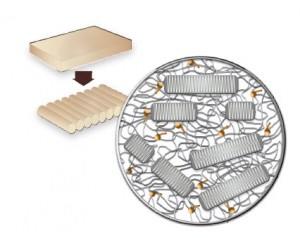
2 Form Bars
Raw material is compression-molded into sheets and machined into bars.

3 Radiation Crosslinking
Gamma radiation is applied, creating crosslinks and some residual free radicals. The vitamin E scavenges some of the free radicals that would otherwise form crosslinks, consequently reducing the crosslinking efficiency of any particular radiation dose.[1] Thus, engineers optimized the radiation dose for E-MAX, ensuring that the crosslink density for hip liners is comparable to 10 Mrad XLPE. For tibial inserts, where more fatigue strength is required, the radiation dose was optimized to achieve a crosslinking density comparable to 7 Mrad XLPE. [2]
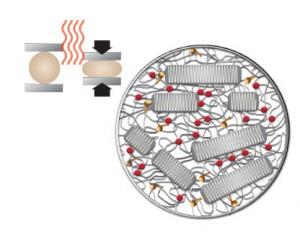
4 Mechanically Anneal
The bars are warmed and then compressed, allowing the residual free radicals to become mobile and combine into additional crosslinks—eliminating free radicals to near the detection threshold.[2,3]
5 Machine, Package, and Sterilize
Components are machined, packaged and EtO-sterilized, thus avoiding the change in material properties or the reintroduction of free radicals associated with gamma sterilization.
- Kurtz S, Bracco P, Costa L. Chapter 16 Vitamin-E-blended UHMWPE biomaterials. In UHMWPE Biomaterials Handbook Second Edition (ed. Kurtz SM). Elsevier: Amsterdam, 2009.
- Materials Characterization testing. Test report TP0322. On file with KYOCERA Medical Technologies, Inc.
- Bhattacharyya S, Matrisciano L, Spiegelberg S, Harris W, Muratoglu O. Mechanical elimination of residual free radicals in an irradiated UHMWPE rod: advantages over melting. 50th annual meeting of the orthopaedic research society. 2004:1474.
Benefits
E-MAX is highly crosslinked to reduce wear and incorporates two new technologies—mechanical-anneal and Vitamin E—which address known problems with melt-annealed XLPE. Laboratory tests have verified the superior oxidative stability and improved mechanical properties of E-MAX.
01 Wear Resistance
The radiation doses for E-MAX hip and knee liners were fine-tuned to achieve crosslinking densities comparable to first-generation XLPE’s, which have demonstrated reduced wear rates both in simulators and in clinical use. [1-10] Simulator studies comparing E-MAX to conventional polyethylene found:
- 89% reduction in hip wear [11]
- 74% reduction in knee wear [11]
02 Mechanical Properties
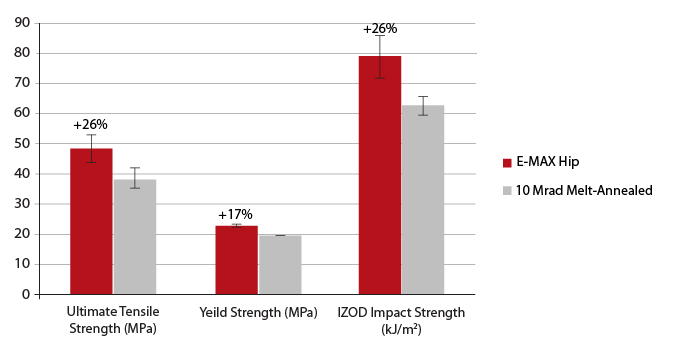
E-MAX is mechanically annealed, eliminating* free radicals without the sacrifice in mechanical properties associated with melt-annealing.[12,13] Laboratory tests have verified the significantly improved strength and toughness of E-MAX compared to melt-annealed XLPE.[14] (Figure 1)
Figure 1: Tensile and IZOD impact test results for E-MAX Highly Crosslinked Polyethylene (Hip). Percentages indicate relative improvement over 10 Mrad melt-annealed XLPE [14].
Fatigue crack propagation analysis comparing E-MAX to melt-annealed XLPE demonstrated:
- E-MAX had a significantly higher stress intensity factor for crack initiation, meaning it is more resistant to crack initiation than melt-annealed XLPE. [15]
- E-MAX had an overall decreased crack propagation rate compared to melt-annealed XLPE. [15]
03 Oxidative Stability
To address the unexpected in vivo oxidation observed in melt-annealed XLPE, E-MAX contains 0.1%-wt vitamin E. Vitamin E is a natural antioxidant that has a stabilizing effect against the oxidation of PE.[16]
The oxidative stability of E-MAX was tested under extreme conditions—samples were exhaustively extracted to remove as much Vitamin E as possible and then subjected to accelerated aging. Even under extreme conditions, no positive oxidation index was measured in E-MAX materials (hip or knee).[15] Furthermore, mechanical testing after aging showed no decrease in tensile or impact strength. [14] (Figure 2)
Figure 2: Artificially-Aged E-MAX Highly Crosslinked Polyethylene (Hip). Strength maintained over 28 days of laboratory aging. [14]

Note that adding vitamin E via the diffusion method (as with Biomet E1®†) typically results in a non-homogeneous, gradient distribution.[17] However, in E-MAX, the vitamin E powder is blended with 1020 UHMWPE resin powder, so the vitamin E is distributed evenly throughout the material.[15]
E1 is a trademark of Biomet, Inc. Warsaw, Indiana.
*With both melt annealing and mechanical annealing, free radicals are eliminated to a level at or near the detection threshold of ESR measurement equipment.
- Kurtz S, Medel FJ, MacDonald D, Rimnac CM. In vivo oxidation, oxidation potential, and clinical performance of highly crosslinked UHMWPEs implanted for up to 8 years. 4th International Meeting UHMWPE for arthroplasty: From Powder to Debris 2009; Torino, Italy.
- Digas G, Karrholm J, Thanner J, Herberts P. 5-year experience of highly cross-linked polyethylene in cemented and uncemented sockets: Two randomized studies using radiostereometric analysis. Acta Orthop 2007; 78(6): 746-54.
- D’Antonio JA, Manley MT, Capello WN, et al. Five-year experience with Crossfire highly cross-linked polyethylene. Clin Orthop Relat Res 2005; 441: 143-50.
- Engh CA, Stepniewski AS, Ginn SD, et al. A randomized prospective evaluation of outcomes after total hip Arthroplasty using crosslinked marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty 2006; 21(6): 17-25.
- Olyslaegers C, Defoort K, Simon JP, Vandenberghe L. Wear in conventional and highly cross-linked polyethylene cups: a 5-year follow-up study. J Arthroplasty 2008; 23(4): 489-94.
- Garcia-Rey E, Garcia-Cimbrelo E, Cruz-Pardos A, Ortega-Chamarro J. New polyethylenes in total hip replacement: a prospective, comparative clinical study of two types of liner. J Bone Joint Surg Br 2008; 90(2): 149-53.
- Geerdink CH, Grimm B, Vencken W, Heyligers IC, Tonino AJ. Cross-linked compared with historical polyethylene in THA: An 8-year clinical study. Clin Orthop Relat Res 2009; 467(4): 979-84.
- Glyn-Jones S, Isaac S, Hauptfleisch J, McLardy-Smith P, Murray DW, Gill HS. Does highly cross-linked polyethylene wear less than conventional polyethylene in total hip arthroplasty? A doubleblind, randomized, and controlled trial using roentgen stereophotogrammetric analysis. J Arthroplasty 2008; 23(3): 337-43.
- Kurtz SM, Medel FJ, MacDonald DW, Parvizi J, Kraay MJ, Rimnac CM. Reasons for revision of first-generation highly cross-linked polyethylenes. J Arthroplasty. 2010 Sep;25(6 Suppl):67-74.
- Kurtz SM. Chapter 8 The clinical performance of UHMWPE in knee replacements. In UHMWPE Biomaterials Handbook Second Edition (ed. Kurtz SM). Elsevier: Amsterdam, 2009.
- University of Nebraska Medical Center. Characterization of CIMA and E-CIMA UHMWPE as a bearing against CoCr femoral hip components: A hip simulation study. Test report dated July 15, 2011. and An in-vitro wear durability study of the Renovis A200 CR Knee System: two sizes and two bearing materials.Test report dated November 11, 2011. On file with KYOCERA Medical Technologies, Inc.
- Wannomae KK, Micheli BR, Lozynsky AJ, Muratoglu OK. A New Method of Stabilizing Irradiated UHMWPE Using Vitamin E and Mechanical Annealing. 11th Congress EFFORT. Madrid, Spain. June 2010.
- Bhattacharyya S, Matrisciano L, Spiegelberg S, Harris W, Muratoglu O. Mechanical elimination of residual free radicals in an irradiated UHMWPE rod: advantages over melting. 50th annual meeting of the orthopaedic research society. 2004:1474.
- Materials Characterization testing. Test report TP0322. On file with KYOCERA Medical Technologies, Inc.
- Cambridge Polymer Group. Analysis of CIMA and E-CIMA Material. Test report dated July 15, 2011. On file with KYOCERA Medical Technologies, Inc
- Costa L, Bracco P. Chapter 21 Mechanisms of crosslinking, oxidative degradation, and stabilization of UHMWPE. In UHMWPE Biomaterials Handbook Second Edition (ed. Kurtz SM). Elsevier: Amsterdam, 2009.
- Gomez-Barrena E, Medel F, Puetrolas JA. Polyethylene oxidation in total hip arthroplasty: evolution and new advances. The Open Orthopedics Journal 2009; 3:115-120.
